Abstract
Introduction: Blinatumomab is a bispecific T-cell engaging antibody that binds and allows CD3 cytotoxic T cells to recognize and eradicate CD-19 positive malignant B cells. Blinatumomab is currently under study for relapsed and refractory B-cell acute lymphoblastic leukemia (RR B-ALL).
Methods: A comprehensive literature search was conducted across various data sets, including PubMed, Cochrane, and Embase, and presented data in ASH and ASCO. A review of the most recent data is summarized in this abstract.
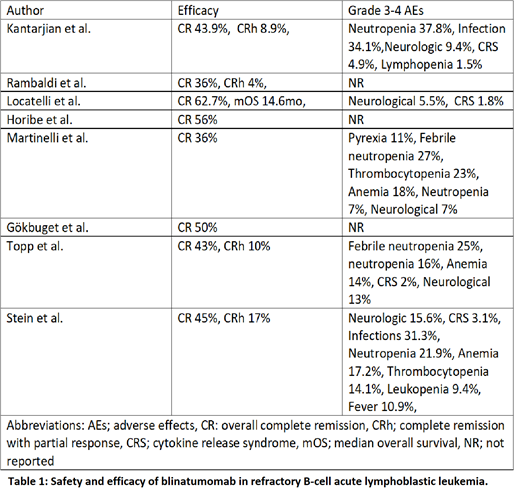
Results: Eight clinical trials are currently under various phases of the study evaluating blinatumomab in RR B-ALL. In the phase 1/2 trial by Locatelli et al., 110 patients treated with blinatumomab showed complete remission (CR) with <5% blast cells of 62.7% at a dose of 5-15 µg/m 2. In phase 1b trial by Horibe et al., nine Japanese children received blinatumomab at a dose of 5 µg/m 2/day for week 1, followed by (f/b) 15 µg/m 2/day for week 2-4 and showed an CR in 56% patients.
In a phase II study, Martinelli et al., treated 45 patients at a dose of 9 µg/day in week 1, f/b 28 µg/day; 36% of the patients achieved CR. In another phase II trial by Gokbuget et al., a 15 µg/day dose in 20 patients resulted CR in 50% pts. Topp et al. reported CR of 43% in 39 patients, and Stein et al. reported 45% CR in 64 patients treated with a similar regimen.
In phase III trials, Rambaldi et al., reported 36% CR in 119 patients and, Kantarjian et al. reported CR of 43.9% in 271 patients treated with blinatumomab at a dose of 9 µg/day during week 1 and 28 µg/d onwards for up to 3 and 6 cycles, respectively.
In all the trials, blinatumomab was given via 4-week continuous IV infusion followed by 2-week treatment-free interval per cycle. The most common grade 3 and 4 adverse effects are listed in the table 1.
Conclusion: Blinatumomab is showing promising results in RR B-ALL with a good side effect profile. However, the final results of these trials are awaited.
Anwer: Janssen pharmaceutical: Honoraria, Research Funding; Allogene Therapeutics: Research Funding; BMS / Celgene: Honoraria, Research Funding; GlaxoSmithKline: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal